atomistica.online
Cell Metabolism
Cell Metabolism
Objective
To explore how living cells obtain, transform, and use energy through metabolic processes. By completing this project, you will understand the importance of cell metabolism for life.
Main elements
Metabolism = the sum of all chemical reactions in the cell.
Catabolism
- Definition: Breakdown of complex molecules into simpler ones, releasing energy.
- Examples:
- Glucose breakdown during cellular respiration (glucose → CO₂ + H₂O + ATP).
- Fat breakdown into fatty acids and glycerol.
- Protein breakdown into amino acids.
Anabolism
- Definition: Building of complex molecules from simpler ones, requiring energy (usually ATP).
- Examples:
- Photosynthesis (CO₂ + H₂O → glucose).
- Protein synthesis from amino acids.
- DNA and RNA synthesis from nucleotides.
ATP
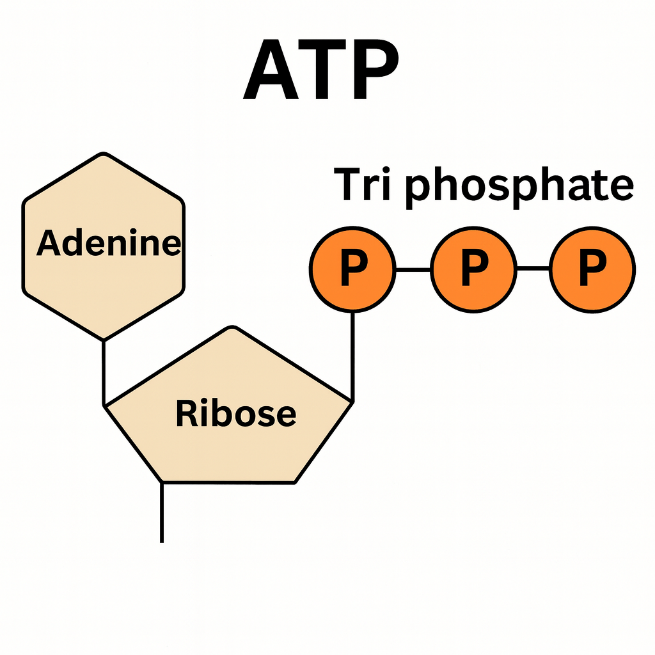
ATP (adenosine triphosphate) = the molecule that stores and transfers energy in the cell.
- Structure: adenine (base) + ribose (sugar) + three phosphate groups.
- Energy is released when one phosphate group is removed (ATP → ADP).
ATP (adenosine triphosphate) has three phosphate groups.
When one phosphate group is split off, ADP (adenosine diphosphate) + energy is formed.
Reaction: ATP → ADP + Pi+ Energy, where Pᵢ = inorganic phosphate
ATP is called the “energy currency” of the cell because it provides energy for vital processes:
- Muscle contraction.
- Protein synthesis.
- Active transport across membranes.
- Driving biochemical reactions.
Metabolic Pathways (examples)
Glycolysis – breakdown of glucose → small amount of energy (ATP)
Definition: Process of breaking down one molecule of glucose (C₆H₁₂O₆) into two molecules of pyruvate.
Location: Cytoplasm of the cell.
Energy yield:
- Produces a small amount of energy – 2 ATP (net gain).
- Produces 2 NADH molecules (carry electrons and energy).
Importance: First step of cellular respiration; provides energy and intermediates for other pathways.
Krebs Cycle (Citric Acid Cycle) – breakdown of molecules, release of CO₂ and energy
Definition: Process of breaking down pyruvate (converted into acetyl-CoA), releasing carbon dioxide.
Location: Mitochondrial matrix.
Energy yield (per one glucose → 2 acetyl-CoA):
- 2 ATP (or GTP),
- 6 NADH and 2 FADH₂ (carry energy and electrons),
- 4 CO₂ (exhaled during respiration).
Importance: Main source of electrons for the electron transport chain, where most ATP is produced.
Cellular Respiration – glucose + O₂ → CO₂ + H₂O + lots of ATP
Definition: Process in which glucose is completely broken down in the presence of oxygen, producing carbon dioxide, water, and a large amount of energy (ATP).
Equation: C6H12O6+6O2 → 6CO2+6H2O+ 36–38 ATP
Location: Mitochondria (cytoplasm for glycolysis, mitochondrial matrix for Krebs cycle, inner mitochondrial membrane for the electron transport chain).
Energy yield:
- Glycolysis → 2 ATP
- Krebs cycle → 2 ATP
- Electron transport chain → ~32–34 ATP
- Total: ~36–38 ATP per glucose molecule.
Importance: The main way energy is obtained in the cells of humans, animals, plants, and most microorganisms.
Photosynthesis – plants make glucose and oxygen from CO₂, water, and light
Definition: Process in which plants, algae, and cyanobacteria use light energy to synthesize glucose from carbon dioxide and water, releasing oxygen.
Equation: 6CO2 + 6H2O + light energy → C6H12O6 + 6O
Location: Chloroplasts (thylakoid membranes and stroma).
Phases of photosynthesis:
- Light reactions – in thylakoids; produce ATP, NADPH, and O₂ (released as a gas).
- Calvin cycle (dark reactions) – in the stroma; use ATP and NADPH to synthesize glucose from CO₂.
Importance:
- Primary source of organic matter and energy on Earth.
Source of oxygen needed for respiration of living organisms
Glycolysis
Glycolysis – the beginning of glucose breakdown in the cytoplasm.
- Krebs Cycle – continuation of breakdown in the mitochondrial matrix, releasing CO₂ and forming NADH/FADH₂.
- Cellular Respiration (Electron Transport Chain) – the final stage in the mitochondrial membrane, where most ATP is produced.
- Photosynthesis – a process in plants, opposite to respiration, where glucose and O₂ are produced.
Therefore:
- Glycolysis and the Krebs cycle are the first two steps of cellular respiration.
- In the diagram they are separated to clearly show the phases individually.
- Together, they are part of the overall cellular respiration (which here is represented as the third step).
Photosynthesis is added to show the connection between plants and animals – what plants produce (glucose and oxygen), animals use for respiration, and vice versa.
Connection to Life
- Without metabolism, there is no energy for life.
- Humans: Energy for muscles and organ function.
- Plants: Growth and nutrient synthesis.
- Animals: Energy for movement and survival.
Conclusion
- Metabolism = the basis of life.
- ATP is the central molecule linking food breakdown to all cellular processes.
Without ATP, cells could not live, grow, divide, or function
Hemoglobin
Heme b
Hemoglobin and Heme B – Oxygen Carriers
Oxygen does not travel through the blood on its own, it is carried by a special protein called hemoglobin in red blood cells.
Each hemoglobin molecule is made of four protein chains, and in each chain there is a heme group.
The heme group contains an iron (Fe²⁺) atom at its center. This iron atom can bind oxygen molecules (O₂) reversibly.
When you breathe in, oxygen from the alveoli binds to hemoglobin in red blood cells. When blood reaches body tissues, hemoglobin releases oxygen for respiration.
The waste product, carbon dioxide (CO₂), travels back to the lungs, either dissolved in plasma, bound to hemoglobin, or as bicarbonate ions.
