atomistica.online
Molecular Basis of Biological Processes
Enzymes and energy in metabolism
1. Introduction
Enzymes are biological catalysts made of proteins that speed up chemical reactions in living organisms. They work by lowering the activation energy needed for a reaction to occur (Figure 1).
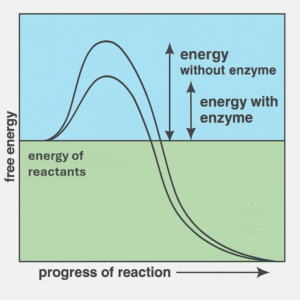
Each enzyme has a specific shape that fits its substrate (the molecule it acts on), similar to a “key and lock” model (Figure 2).
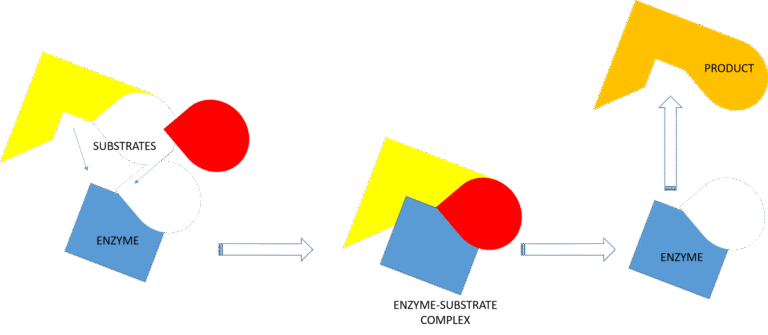
The enzyme binds to the substrate to form an enzyme–substrate complex, facilitates the reaction, and then releases the products while remaining unchanged and ready to catalyze another reaction
The role of coenzymes and examples of important ones (e.g. NAD⁺, FAD, CoA)
Coenzymes are small, non-protein molecules that help enzymes perform their catalytic functions.
They often act as carriers of electrons, atoms, or chemical groups during biochemical reactions. Examples include NAD⁺ (nicotinamide adenine dinucleotide) and FAD (flavin adenine dinucleotide), which transfer electrons in cellular respiration, and CoA (coenzyme A), which carries acyl groups in energy metabolism.
Coenzymes are small, non-protein molecules that help enzymes perform their catalytic functions.
They often act as carriers of electrons, atoms, or chemical groups during biochemical reactions. Examples include NAD⁺ (nicotinamide adenine dinucleotide) and FAD (flavin adenine dinucleotide), which transfer electrons in cellular respiration, and CoA (coenzyme A), which carries acyl groups in energy metabolism.
Figure 3 illustrates the formation of a functional enzyme (holoenzyme) and its interaction with a substrate:
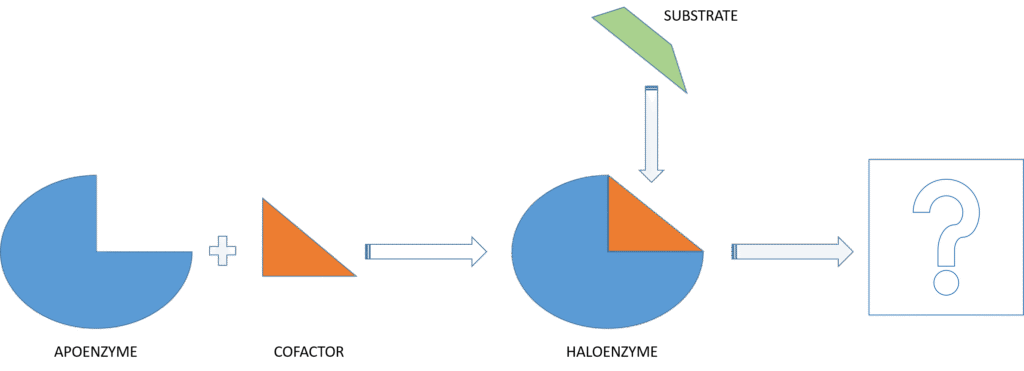
- Apoenzyme (blue shape)
- An inactive enzyme protein shown as a blue shape with a missing part.
- It cannot function without its cofactor.
- Cofactor (orange triangle)
- A non-protein molecule (can be a metal ion or an organic coenzyme) that binds to the apoenzyme.
- Its binding is essential for enzyme activation.
- Holoenzyme (blue + orange)
- The active enzyme form created when the apoenzyme binds with the cofactor.
- Only the holoenzyme can interact with the substrate.
- Substrate (green shape)
- The molecule upon which the enzyme acts.
- It fits into the active site of the holoenzyme, enabling the catalytic reaction.
- Reaction Product (missing – marked with a question mark)
- The diagram ends with a question mark, indicating that the final product of the enzymatic reaction is missing.
- To complete the diagram, a drawing representing the product of the enzyme-substrate reaction should be added.
How enzymes affect the rate of metabolic reactions
Enzymes significantly increase the rate of metabolic reactions by providing an alternative reaction pathway with a lower activation energy. Without enzymes, most biochemical reactions in living organisms would occur too slowly to sustain life.
Several factors influence the rate of enzyme activity:
- Enzyme concentration
When sufficient substrate is available, increasing the enzyme concentration increases the reaction rate, as more active sites are available for substrate binding.
- Substrate concentration
At a constant enzyme concentration, increasing the substrate concentration initially increases the rate of reaction. However, once all enzyme active sites are occupied, the enzymes become saturated, and the reaction rate reaches a maximum level.
- Temperature
Each enzyme has an optimum temperature at which it functions most effectively. As temperature increases, molecular motion and collision frequency increase, enhancing enzyme activity. However, excessive heat denatures enzymes by disrupting their hydrogen bonds and altering their three-dimensional structure. Low temperatures slow down enzyme activity by reducing molecular motion.
- pH
Each enzyme has an optimal pH that maintains its functional shape. Deviations from this pH can change the enzyme’s charge and disrupt ionic bonds, leading to denaturation and loss of catalytic activity.
- Salt concentration
Enzymes also have an optimal salt concentration. Significant changes in ionic strength can denature enzymes and reduce their effectiveness.
Environmental and chemical factors can also affect enzyme activity in microorganisms. Many disinfectants, such as chlorine, iodine, iodophores, mercurials, silver nitrate, formaldehyde, and ethylene oxide inactivate bacterial enzymes and thereby block metabolic processes. Similarly, high temperatures (autoclaving, boiling, pasteurization) denature enzymes, while low temperatures (refrigeration, freezing) slow down or temporarily halt enzyme-catalyzed reactions.
Under optimal conditions of temperature, pH, and concentration, enzyme activity is at its maximum, and the rate of metabolic reactions is highest.
How energy is produced and used in cells (ATP cycle)
Cells produce energy mainly in the form of ATP (adenosine triphosphate) through processes like cellular respiration.
ATP acts as the energy currency of the cell. When the third phosphate group is broken off (ATP → ADP + P), energy is released and used for vital activities such as muscle contraction, active transport, and biosynthesis. Enzymes control both the production and use of ATP to ensure efficiency (Figure 4).
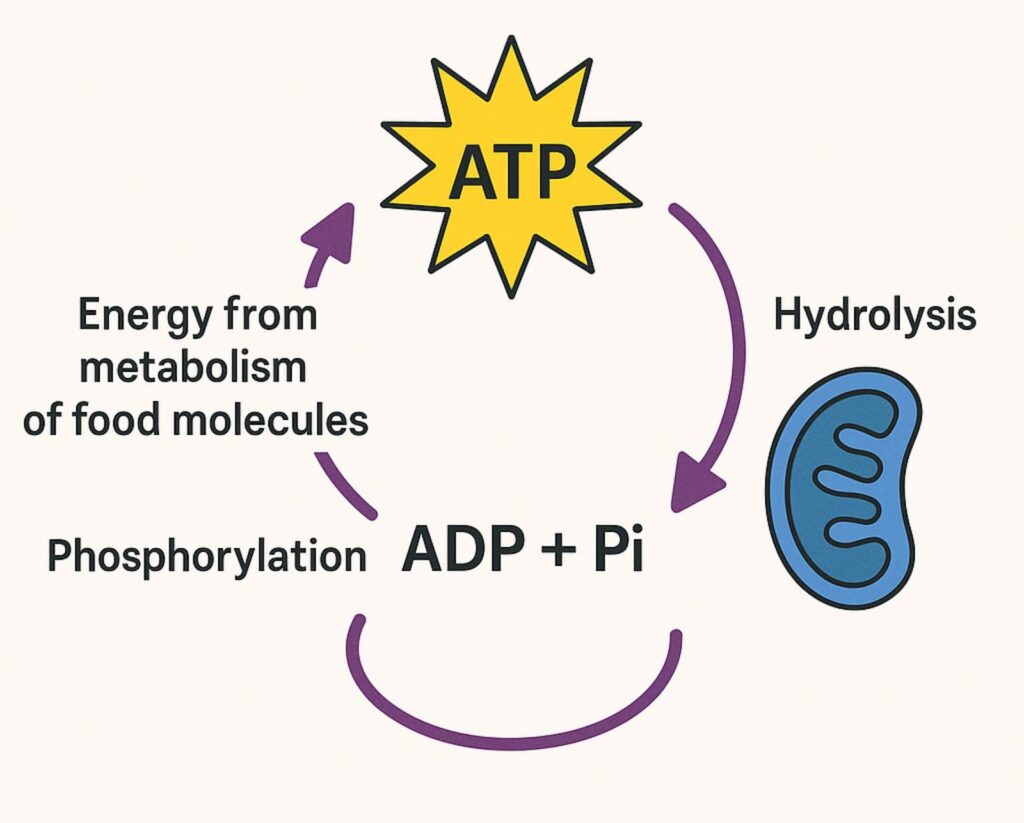
Hydrolysis
ATP is broken down into ADP + Pi (inorganic phosphate).
This reaction releases energy for cellular processes (e.g., muscle contraction, active transport, biosynthesis).
Phosphorylation
ADP is converted back into ATP by adding a phosphate group (Pi).
This process requires energy, which comes from the metabolism of food molecules.
Connection of enzymes with energy processes in metabolism (catabolism and anabolism)
In catabolic reactions, enzymes break down complex molecules (like glucose) into simpler ones, releasing energy that is captured as ATP.
In anabolic reactions, enzymes help build complex molecules (like proteins or DNA) using the energy stored in ATP.
Thus, enzymes are central to both energy release and energy consumption in living cells, maintaining the continuous flow of energy through metabolism.
